Alumina Trihydrate vs Aluminum Hydroxide: Key Differences, Properties & Applications
Date: August-06-2025 Categories: News、Aluminium Hydroxide Views: 416
Alumina Trihydrate (ATH) and Aluminum Hydroxide are closely related materials that share the same chemical formula but differ in their industrial terminology, crystal forms, and applications. Understanding their distinctions is essential for selecting the right material for flame retardant fillers, polymer additives, and high-performance industrial uses.

Chemical Definition and Relationship
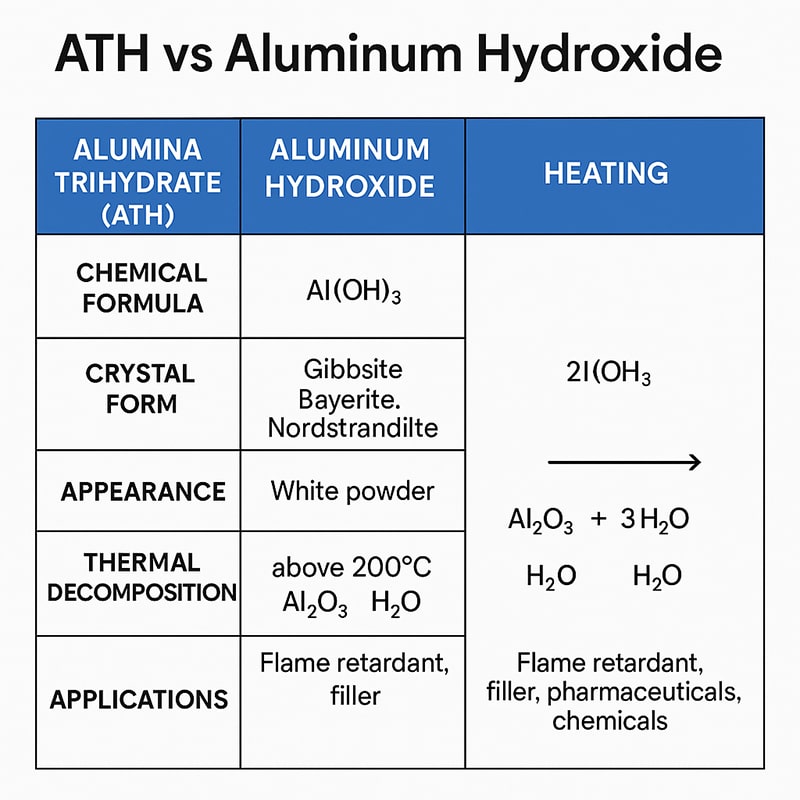
Alumina Trihydrate (ATH): ATH is a crystalline form of Al(OH)₃, primarily existing as gibbsite. It is the most common commercial form of aluminum hydroxide, widely used in flame retardant and filler applications.
Aluminum Hydroxide: Aluminum hydroxide powder is a broader term that includes ATH and other less common crystal forms such as bayerite and nordstrandite. Essentially, ATH is a specific industrial grade of aluminum hydroxide optimized for fillers and flame retardant applications.
In short: All ATH is aluminum hydroxide, but not all aluminum hydroxide is ATH.
Crystal Structure and Physical Properties
| Property | Alumina Trihydrate (ATH) | Aluminum Hydroxide |
|---|---|---|
| Chemical Formula | Al(OH)₃ | Al(OH)₃ |
| Crystal Form | Gibbsite | Gibbsite, Bayerite, Nordstrandite |
| Appearance | White powder, high whiteness, controlled particle size | Powder, crystalline or colloidal form |
| Thermal Stability | Decomposes above 200°C to form alumina | Same behavior but may vary slightly by crystal form |
| Processing | Surface modification for polymer flame retardants | Used for chemical, pharmaceutical, and industrial applications |
Which One Should You Choose? (Application-Based Comparison)
Although alumina trihydrate (ATH) and aluminum hydroxide share the same chemical formula, their performance differs significantly depending on application requirements. Choosing the right material depends on temperature resistance, flame-retardant efficiency, and cost-performance balance.
Flame-Retardant Plastics and Cables
For flame-retardant plastics, rubber compounds, and cable insulation, alumina trihydrate (ATH) is usually the preferred choice. ATH releases water at relatively low temperatures, which helps suppress smoke and reduce heat during combustion.
High-Temperature or Chemically Stable Applications
In applications requiring higher thermal stability or chemical purity, aluminum hydroxide with controlled particle size may be more suitable, especially when flame retardancy is not the primary requirement.
Cost vs Performance Considerations
ATH is widely used in large-volume industrial applications due to its cost efficiency and proven flame-retardant performance. Aluminum hydroxide may be selected for specialized uses where formulation flexibility or downstream processing is more critical.
In most flame-retardant and filler applications, alumina trihydrate remains the first-choice material, while aluminum hydroxide serves as an alternative for specific technical requirements.
Key Differences Between Alumina Trihydrate and Aluminum Hydroxide
| Aspect | Alumina Trihydrate (ATH) | Aluminum Hydroxide |
|---|---|---|
| Chemical formula | Al(OH)3 | Al(OH)3 |
| Common industrial name | ATH (flame-retardant filler) | Aluminum hydroxide |
| Decomposition temperature | ~220°C | ~300°C |
| Flame-retardant effect | Excellent smoke suppression | Moderate |
| Typical applications | Cables, plastics, rubber, flame-retardant composites | Chemicals, pharmaceuticals, specialty fillers |
| Cost-performance ratio | High for large-scale industrial use | More application-specific |
Production Process
Both ATH and aluminum hydroxide are mainly produced through the Bayer process:
- Dissolution of bauxite in caustic soda to form sodium aluminate;
- Precipitation of aluminum hydroxide from the solution;
- Classification, drying, and surface treatment to produce ATH;
- Optional calcination for producing calcined alumina.
Main Applications
| Application | Alumina Trihydrate (ATH) | Aluminum Hydroxide |
|---|---|---|
| Flame Retardant | Widely used in cables, rubber, plastics, LSZH compounds | Same, ATH dominates this segment |
| Filler | Artificial stone, coatings, adhesives | Same applications |
| Electronics & Ceramics | High-purity ATH as a precursor for alumina | Same |
| Pharmaceuticals | — | Antacids and medical-grade hydroxide |
| Chemicals | — | Used as a chemical reagent and catalyst precursor |
Thermal Decomposition and Flame Retardancy
ATH decomposes at approximately 200°C according to the following reaction:
2Al(OH)₃ → Al₂O₃ + 3H₂O↑
This process provides several flame-retardant effects:
- Endothermic reaction: Absorbs heat, lowering the material temperature.
- Water vapor release: Dilutes flammable gases and oxygen concentration.
- Protective alumina layer: Forms a barrier on the material surface, improving fire resistance.
This is why ATH is the material of choice for low-smoke halogen-free (LSZH) compounds and high-performance flame retardant systems.
Key Differences at a Glance
- ATH is the most common commercial form of aluminum hydroxide, mainly gibbsite.
- Aluminum hydroxide is a broader term that includes ATH and other crystal forms.
- ATH is optimized for flame retardant and filler applications, while aluminum hydroxide has additional uses in pharmaceuticals and chemical industries.
- Both decompose to alumina at high temperatures, but ATH is specifically designed for industrial flame retardancy.
Conclusion
Alumina Trihydrate (ATH) and Aluminum Hydroxide share the same chemical composition but differ in their industrial relevance. For cable compounds, plastics, coatings, and rubber, ATH is the preferred material. For pharmaceuticals and chemical processing, other grades of aluminum hydroxide may be more suitable.
For industrial-grade alumina trihydrate used in flame-retardant and filler applications, you can explore our alumina trihydrate product specifications for detailed technical data.
Banlanchem supplies high-purity ATH and aluminum hydroxide with customized particle size and surface treatment to meet specific industrial requirements.















