What Is the Difference Between Aluminum and Aluminium? (Complete Alumina vs Aluminum Guide)
Across different regions, people spell the same metal in two ways — aluminum and aluminium. But when the discussion shifts to alumina vs aluminum, we are no longer talking about spelling. We are talking about two totally different materials used for very different industrial purposes.
This extended guide is written for engineers, buyers, wholesalers, factories, and anyone looking for a clear, practical understanding of how aluminum, aluminium, and alumina differ. It is provided by BanlanChem, a global manufacturer, supplier, and factory specializing in alumina-based materials for ceramics, catalysts, chemicals, fillers, and advanced applications.
Table of Contents
- 1. Aluminum vs Aluminium: Why Two Spellings?
- 2. Quick Summary: Alumina vs Aluminum
- 3. Chemical Identity: What Each Material Really Is
- 4. Physical Properties Compared
- 5. Chemical Behavior and Stability
- 6. How Each Material Is Made
- 7. Industrial Applications (Metal vs Ceramic)
- 8. Why They Cannot Replace Each Other
- 9. Comparison Table: Alumina vs Aluminum
- 10. FAQ
- 11. About BanlanChem – Manufacturer, Supplier, Factory, Wholesale
1. Aluminum vs Aluminium: Why Two Spelling Versions Exist
The difference between aluminum and aluminium is purely regional spelling. Both words refer to the same metallic element with the chemical symbol Al. The origin of the difference dates back to early chemical naming standards:
- Aluminium follows the traditional naming system used in Europe.
- Aluminum became the preferred American spelling after the early 1900s.
In industrial practice, both spellings appear in technical papers, safety data sheets, supplier catalogs, and international standards. However, regardless of the spelling, they always refer to the same material — metallic aluminum.
But alumina is not the same thing. Let’s explain clearly.
2. Quick Summary: Alumina vs Aluminum (Simple Explanation)
If you remember only one part of this article, let it be this:
- Aluminum / Aluminium (Al) → soft metal, conductive, shiny, lightweight, reactive.
- Alumina (Al₂O₃) → hard white ceramic, extremely stable, high melting point, non-conductive.
Alumina is created when aluminum reacts with oxygen and forms a stable oxide. This oxide layer is usually only a thin film on the surface of aluminum metal, but when produced intentionally, alumina becomes its own industrial ceramic material.
That is why alumina is used for abrasives, catalysts, ceramics, refractories, insulation, and fillers — nothing like metal aluminum.
3. Chemical Identity: What Each Material Really Is
Aluminum / Aluminium (Al)
Elemental aluminum is a metal. It has metallic bonding, free-moving electrons, and excellent electrical and thermal conductivity. Because it reacts immediately with air, aluminum always forms a thin alumina layer on its surface.

Alumina (Al₂O₃)
Alumina, or aluminum oxide, is a ceramic. The aluminum atoms are chemically bonded with oxygen atoms, forming a strong crystal lattice. This structure:
- does not conduct electricity,
- is stronger than steel in compression,
- withstands temperatures above 2000°C,
- resists chemical attack,
- remains stable in harsh conditions.
This transformation from metal to ceramic completely changes the material category.
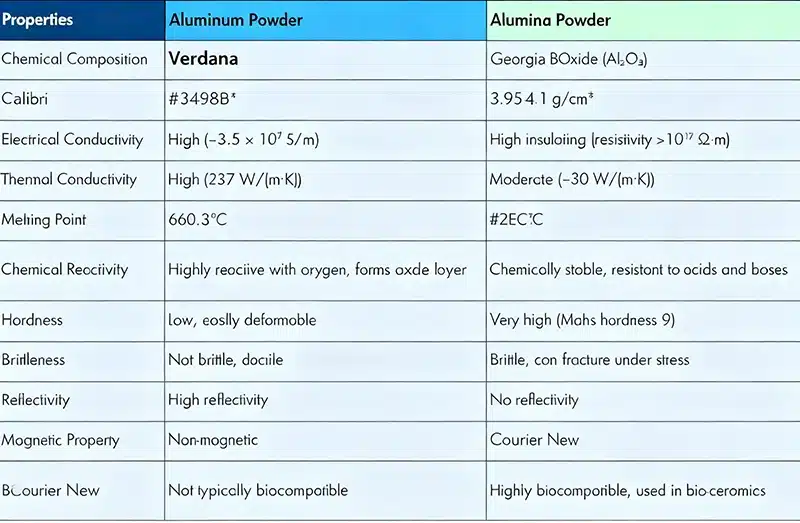
4. Physical Properties Compared
| Property | Aluminum (Al) | Alumina (Al₂O₃) |
|---|---|---|
| Appearance | Silver-gray, metallic shine | White, matte, ceramic-like |
| Electrical Conductivity | High | None (insulator) |
| Density | ~2.7 g/cm³ | ~3.9 g/cm³ |
| Hardness | Soft | Mohs hardness ~9 |
| Melting Point | 660°C | 2050°C |
| Chemical Stability | Reactive | Highly stable |
| Safety | Flammable as fine powder | Non-flammable |

These differences explain why industries specifically choose alumina for ceramic applications and aluminum for metallic applications.
5. Chemical Behavior and Stability
Aluminum / Aluminium
- Forms an oxide layer instantly in air
- React with acids and bases to release hydrogen gas
- Can participate in thermite reactions
- Powder can ignite or explode in oxygen-rich environments
Alumina
- Does not burn or explode
- Resists corrosion and most chemicals
- Stable at extremely high temperatures
- Used as a catalyst support in petrochemical processes
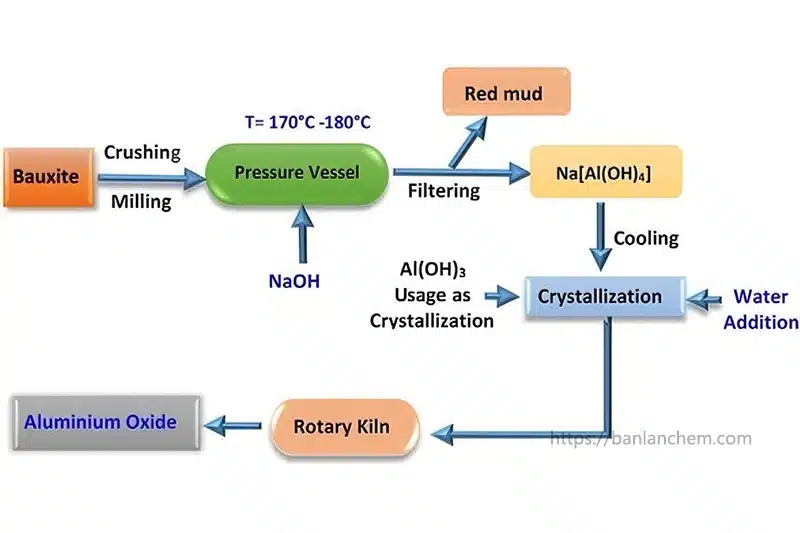
6. How Each Material Is Made
How Alumina Is Produced
The major industrial method is the Bayer Process:
- Bauxite is crushed.
- Aluminum hydroxide is extracted.
- The hydroxide is calcined at high temperature.
- Alumina powder is formed.
You can find BanlanChem’s alumina-related products here:
- High Purity Alumina Powder
- Aluminum Hydroxide Filler Grade
- Pseudoboehmite for Catalysts
- Boehmite Alumina
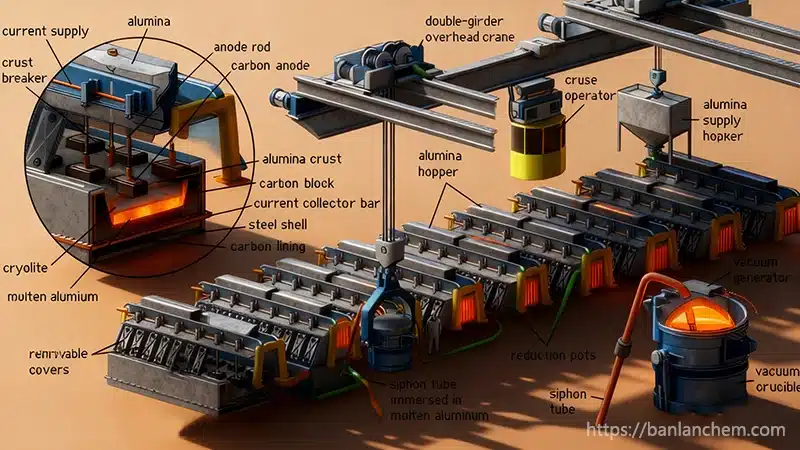
How Aluminum Powder Is Produced
- Atomization: molten aluminum is broken into fine droplets.
- Ball milling: grinding solid aluminum into powder.
- Centrifugal spraying: spinning molten aluminum to form particles.
7. Industrial Applications (Metal vs Ceramic)
Applications of Aluminum (Metal)
- Fireworks and explosives
- Conductive coatings
- Thermite welding
- Metal pigments for paints
- Alloy production

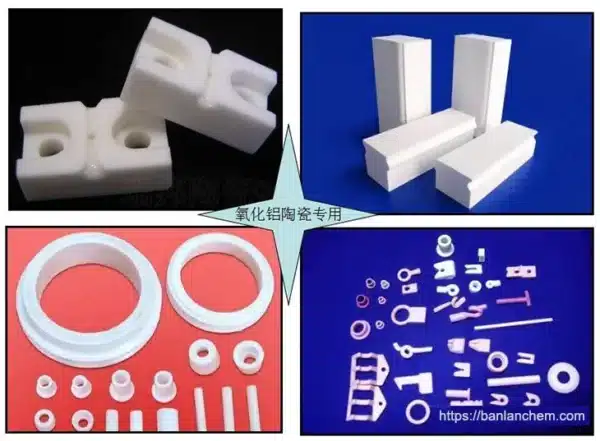
Applications of Alumina (Ceramic)
- Abrasives and polishing materials
- Refractory bricks, kiln linings
- Ceramic substrates and insulators
- Filler for plastics and rubber
- Petrochemical catalyst carriers
- Adsorbents and filtration media
Further reading:
8. Why Aluminum and Alumina Cannot Replace Each Other
Although alumina is made from aluminum, the two materials perform different roles and cannot be substituted.
- You cannot use aluminum powder for abrasives — it’s too soft.
- You cannot use alumina in fireworks — it does not react.
- Aluminum melts too easily to be used as a refractory.
- Alumina does not conduct electricity, so it cannot replace metal aluminum.
Every professional manufacturer and supplier clearly separates the two categories during production and industrial application.
9. Comparison Table: Alumina vs Aluminum
| Feature | Aluminum (Metal) | Alumina (Ceramic) |
|---|---|---|
| Material Type | Elemental metal | Oxide ceramic |
| Conductivity | High | Insulator |
| Hardness | Soft | Very hard |
| Chemical Reactivity | High | Very low |
| Melting Point | Low (660°C) | Very high (>2000°C) |
| Safety | Flammable as powder | Safe and stable |
10. FAQ
Is aluminum the same as alumina?
No. Aluminum is a metal. Alumina is a ceramic oxide.
Why do some countries spell it “aluminium”?
British chemical naming tradition. The USA shortened the spelling to “aluminum.”
Which is harder, aluminum or alumina?
Alumina is much harder — almost as hard as diamond.
Can aluminum powder explode?
Yes. Fine aluminum particles can ignite under the right conditions.
Is alumina safe?
Yes. Alumina is non-toxic, non-flammable, and widely used in medical, chemical, and industrial applications.
11. About BanlanChem – Manufacturer, Supplier, Factory, Wholesale

Linyi Banlan Chemical Technology Co., Ltd. is a national high-tech enterprise specializing in alumina-based materials. We provide a full range of products, including:
- Alumina powder
- Aluminum hydroxide (ATH)
- Pseudoboehmite
- Boehmite alumina
- Activated alumina
- 4A zeolite

We support OEM/ODM customization, large-volume wholesale, stable supply, and global shipment.
If you are searching for a reliable alumina manufacturer, supplier, or factory partner, feel free to contact us for technical data, samples, or pricing.














