Why Alumina Has High Melting Point? (Aluminum Oxide, Al2O3)
Why Alumina Has High Melting Point? This question matters for ceramics, refractories, polishing, and electronics. Alumina (also called aluminum oxide, Al2O3) stays stable at very high heat. It does not soften easily. This is why many engineers choose it.
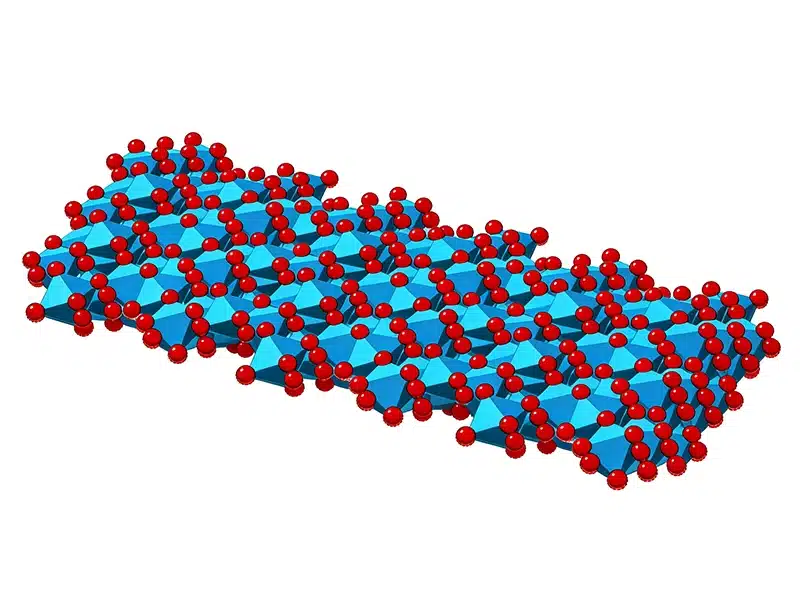
If you buy alumina for industrial use, you may also care about stable quality, particle size control, and batch consistency. BanlanChem is a Manufacturer, Supplier, and Factory that supports Wholesale supply for global buyers.
Contents
- Quick Answer: Why Alumina Has High Melting Point?
- What Is Alumina (Al2O3)?
- Reason 1: Strong Ionic-Covalent Bonding
- Reason 2: Dense Crystal Lattice (Corundum)
- Reason 3: High Lattice Energy
- Reason 4: Stable Structure at High Temperature
- Alumina vs Other Oxides: Melting Point Comparison
- What High Melting Point Means in Real Applications
- How to Choose Alumina for High-Heat Use
- FAQ
- Related Pages on BanlanChem
Quick Answer: Why Alumina Has High Melting Point?
Why Alumina Has High Melting Point? Because the atoms in alumina are held together by very strong bonding in a tight crystal structure. To melt alumina, you must break a large number of strong bonds. That needs a lot of energy, so the melting point is very high.
- Strong bonding between aluminum and oxygen
- Dense lattice (corundum structure for alpha alumina)
- High lattice energy means high heat is needed to melt
- Stable at high temperature with low chemical breakdown
So, when people ask “Why Alumina Has High Melting Point?” the simple answer is: strong bonds + dense structure + high energy needed.
What Is Alumina (Al2O3)?
Alumina is aluminum oxide with the formula Al2O3. It can exist in several forms (phases). The most common high-temperature stable form is alpha alumina (also called corundum). In many industrial products, alumina is used as:
- A refractory raw material for high-temperature linings
- A ceramic material for wear resistance and insulation
- A polishing material for surface finishing
- A filler or functional powder in special materials
Different grades have different particle sizes and purity. For example, fine alumina powders are often used for polishing, while other sizes are used in refractories and ceramics.
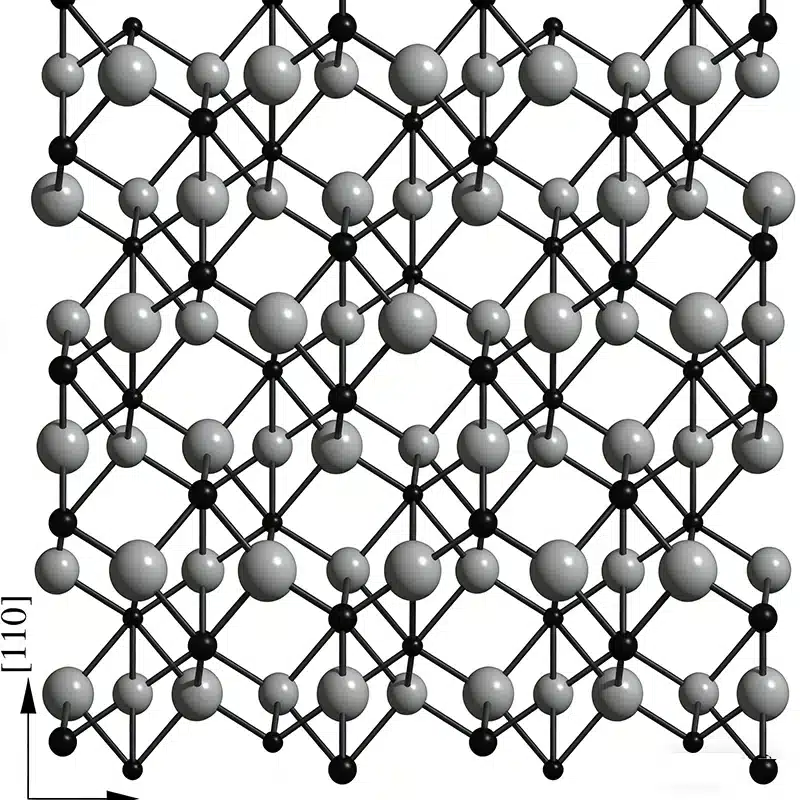
Reason 1: Strong Ionic-Covalent Bonding
Why Alumina Has High Melting Point? The first key reason is the bonding type inside Al2O3.
Alumina has a mix of ionic bonding and covalent character:
- Aluminum atoms tend to form Al3+
- Oxygen atoms tend to form O2−
- Opposite charges attract strongly (ionic part)
- There is also electron sharing (covalent part)
This bonding is very strong. Strong bonds mean you need more heat to break them. That directly supports a high melting point.
Visual idea: Think of it like a very strong “lock” between Al and O. Heating must be extreme to unlock it everywhere in the crystal.
Reason 2: Dense Crystal Lattice (Corundum)
Why Alumina Has High Melting Point? Another reason is the crystal structure, especially for alpha alumina.
Alpha alumina has a dense and stable lattice. The ions are packed tightly. Tight packing gives:
- High mechanical strength
- High hardness
- High resistance to softening
- High melting point
When a material has a dense lattice, melting becomes harder. The structure does not “open up” easily. It holds its form until very high temperature.
Reason 3: High Lattice Energy
Why Alumina Has High Melting Point? Because alumina has high lattice energy.
Lattice energy is the energy that holds ions together in a crystal. In alumina:
- The charges are high (Al3+ and O2−)
- The attraction is strong
- The total “hold force” of the crystal is large
High lattice energy means you must add a lot of heat to separate the ions. That is why alumina needs very high temperature to melt.
Reason 4: Stable Structure at High Temperature
Why Alumina Has High Melting Point? Alumina is also stable at high temperature from a chemical point of view.
- It does not decompose easily
- It has good oxidation resistance
- It keeps stable phases at high heat (especially alpha alumina)
In many industrial furnaces, materials fail because they react, decompose, or soften. Alumina resists these problems, so it can survive where other materials fail.
Alumina vs Other Oxides: Melting Point Comparison
Why Alumina Has High Melting Point? A quick way to understand it is to compare alumina with other oxides used in industry.
- Alumina (Al2O3): very high melting point and strong structure
- Silica (SiO2): high melting point too, but structure and softening behavior differ
- Magnesia (MgO): also high melting point, used in refractories
- Calcia (CaO): high melting point, but reacts easily with water and CO2
Alumina stands out because it balances high melting point, high hardness, and chemical stability. That is why it is common in ceramics and refractories.
What High Melting Point Means in Real Applications
When people ask “Why Alumina Has High Melting Point?” they often want to know what it means for real use. Here are the main benefits:
1) Refractories and furnace linings
- Works in high-temperature kilns and furnaces
- Helps improve lining life
- Resists thermal shock better when designed correctly
2) Advanced ceramics
- Good for high-heat electrical insulation
- Good wear resistance at high temperature
- Stable dimension at heat compared with many plastics
3) Polishing and surface finishing
- Hard material can cut and polish surfaces
- Stable under friction heat during polishing
4) Electronics and thermal management
- Used in substrates and insulating parts
- Handles heat cycles without easy deformation
In short: alumina’s high melting point supports long life in hot environments.
How to Choose Alumina for High-Heat Use
Why Alumina Has High Melting Point? The “why” is the science. The “how to choose” is the buying decision. If you buy alumina for refractories or ceramics, focus on these practical points:
- Purity: higher purity often supports better high-temperature performance
- Phase: alpha alumina is the most stable at high temperature
- Particle size (PSD): affects sintering, packing, and final strength
- Impurities: some impurities can lower softening temperature or create glassy phases
- Consistency: stable batches reduce production risk
If you need stable industrial supply, work with a reliable Manufacturer and Supplier. BanlanChem supports Factory direct and Wholesale orders for alumina products, with export documents such as COA/MSDS when needed.
FAQ
FAQ 1: What is the melting point of alumina?
Alumina has a very high melting point (often reported around the 2000°C range). Exact values can vary by source and conditions, but it is widely known as a high-melting-point oxide.
FAQ 2: Why Alumina Has High Melting Point compared with many metals?
Why Alumina Has High Melting Point? Because its ionic-covalent bonds in a tight lattice need more energy to break than the metallic bonds in many common metals.
FAQ 3: Does higher purity alumina always mean higher melting point?
The melting point of the base compound is a material property, but impurities can make a material soften or react earlier in real service. So higher purity often performs better at high temperature in practical use.
FAQ 4: Is alumina the same as aluminum oxide?
Yes. Alumina is the common industrial name for aluminum oxide (Al2O3).
FAQ 5: Can alumina be used in high-temperature applications?
Yes. That is one of its main uses. It is common in refractories, ceramics, and high-heat insulation parts.
Related Pages on BanlanChem
- Alumina Powder
- Calcined Alumina (High-Temperature & Industrial Grade)
- Aluminum Hydroxide / ATH (Related Raw Material)
Why Alumina Has High Melting Point? Because alumina is built like a “strong crystal fortress”: strong bonding, dense lattice, and high lattice energy. If you need stable alumina powder supply for ceramics, refractories, or polishing, BanlanChem can support you as a Manufacturer, Supplier, and Factory for Wholesale orders.














