What Is the Difference Between Boehmite and Pseudo-Boehmite?
Alumina, also known as aluminum oxide (Al₂O₃), is a widely used material in many industries around the world. It comes in two major types: metallurgical-grade alumina and non-metallurgical alumina (also called chemical alumina or specialty alumina).
Chemical alumina includes many different forms such as pseudo-boehmite, boehmite, ultrafine aluminum hydroxide, activated alumina, calcined alumina, and Tabular Alumina. In this article, we’ll focus on pseudo-boehmite and boehmite, which are two closely related but distinct materials.

What Are Boehmite and Pseudo-Boehmite?
Boehmite (γ-AlOOH)
Boehmite, also known as monohydrate soft alumina or bauxite boehmite, is a well-crystallized form of aluminum oxyhydroxide with a clear and stable structure. It is an important precursor for producing activated alumina, and it is more stable than pseudo-boehmite due to its higher degree of crystallinity.
Pseudo-Boehmite
Pseudo-boehmite looks similar to boehmite in terms of chemical composition, but it has an incomplete crystal structure. It contains 1.25–2.0 molecules of water and is also known as monohydrate alumina or pseudo-monohydrate soft alumina.

Its structure consists of ultra-thin, folded layers, giving it a special porous network. This structure gives pseudo-boehmite a high surface area, large pore volume, and unique thixotropic behavior in acidic environments.
Main Applications
Applications of Boehmite
Boehmite can withstand high processing temperatures, making it ideal as a flame retardant in halogen-free copper-clad boards, electronic insulation, and plastic housings.
It is also a primary raw material for producing activated alumina catalysts used in the coal chemical industry. Furthermore, boehmite is used in:
- Battery separators for lithium and sodium sulfur batteries
- Calcined alumina production
- Ceramics, including microcrystalline, precision, and foam ceramics
- Semiconductors, such as IC chips
- Aerospace lighting components
Especially, low-sodium ultrafine boehmite can be used to make high-purity alumina with excellent insulation, sintering, and transformation properties.
Applications of Pseudo-Boehmite
Pseudo-boehmite’s high surface area and porous structure make it ideal for:
- Catalysts, carriers, and binders in the petroleum industry
- Catalytic coatings for vehicle exhaust systems
- Flame retardant additives in firefighting materials
- Ink-absorbing layers in high-end inkjet paper
- Gas purification adsorbents and fluoride removers in water treatment
- Color and odor removers in industrial wastewater
- Paint additives in construction
- Reinforcing fillers in ceramic composites
Relationship and Differences Between the Two
Although both materials share the same molecular formula (AlOOH), they are different crystalline forms of monohydrate alumina.
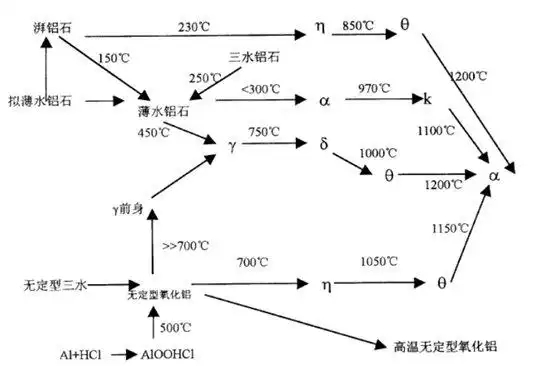
Pseudo-boehmite can transform into boehmite under certain hydrothermal conditions. The two materials look very similar in X-ray diffraction (XRD) patterns, but boehmite has stronger and sharper diffraction peaks due to better crystallinity.
Typically, if the average crystal size is:
- <10 nm: it’s considered pseudo-boehmite
- >50 nm: it’s considered boehmite
- 10–50 nm: may be classified as intermediate (often still called pseudo-boehmite)
Summary of Key Differences
- Structure: Pseudo-boehmite is an amorphous material with weak diffraction peaks. Boehmite has a better crystal structure with sharp diffraction peaks.
- Surface Properties: Pseudo-boehmite has a higher surface area and larger pore volume. Its surface area decreases with temperature, while boehmite’s surface area increases first, then decreases.
How Are They Made?
Producing Pseudo-Boehmite
There are many methods to produce pseudo-boehmite, depending on raw materials and desired product quality. Common methods include:

- Flash precipitation from gibbsite (Al(OH)₃)
- Neutralization using aluminum salts
- Alcohol-aluminum method
- Alkali methods
- Nitric acid method
The process typically includes gel formation, aging, washing, drying, and shaping. Because pseudo-boehmite has very fine particles, careful control is required during washing and drying.
Abroad, the alcohol-aluminum method is widely used due to its high product purity, although it's energy-intensive and expensive.
In China, neutralization methods (acid or alkali-based) are more common, but they face challenges like pollution and inconsistent product quality.
A carbonation method has the lowest cost, but controlling the conditions to produce high-quality pseudo-boehmite is difficult.
Therefore, the industry is seeking new production technologies that are energy-efficient, eco-friendly, and capable of delivering stable product quality.
Producing Boehmite
Boehmite is typically made from aluminum salts through neutralization or from aluminum alcohol salts through hydrolysis.

Some research examples include:
- Using Al(NO₃)₃·6H₂O and sodium citrate in a water-acetone system to create hollow boehmite spheres.
- Using AlCl₃·6H₂O in ethylene glycol to make fiber-like boehmite particles.
- Reacting AlCl₃·6H₂O in an ethanol-water mixture to form 3D “urchin-like” structures.
Direct hydrolysis of aluminum powder can also be used, but the resulting aluminum hydroxide is often multi-phase and lacks the crystal purity needed for high-grade alumina.
Among all techniques, hydrothermal synthesis is gaining popularity due to its simplicity, controllability, and environmental benefits. It is considered a promising method for sustainable alumina production.
Final Thoughts
Even though pseudo-boehmite and boehmite have similar chemical compositions, they differ significantly in terms of crystallinity, physical and chemical properties, manufacturing methods, and end-use applications.
Pseudo-boehmite is best for adsorption and catalyst support because of its high surface area and reactivity.
Boehmite, with better crystallinity and heat resistance, is ideal for producing high-purity alumina.
Understanding the differences between these two materials helps industries select the right alumina precursor for specific high-performance applications.














